Vidac Pharma offers a breakthrough new mode of action to treat cancer, targeting a common characteristic of cancer cells, reverting their abnormal metabolism. Vidac’s group of new chemical entities is based on a dominant IP portfolio of seven patent families. Its pipeline of novel therapies offers efficacy and tolerability to dermatologists, oncologists, and their patients.
First-in-Class Portfolio in Dermatology and Oncology
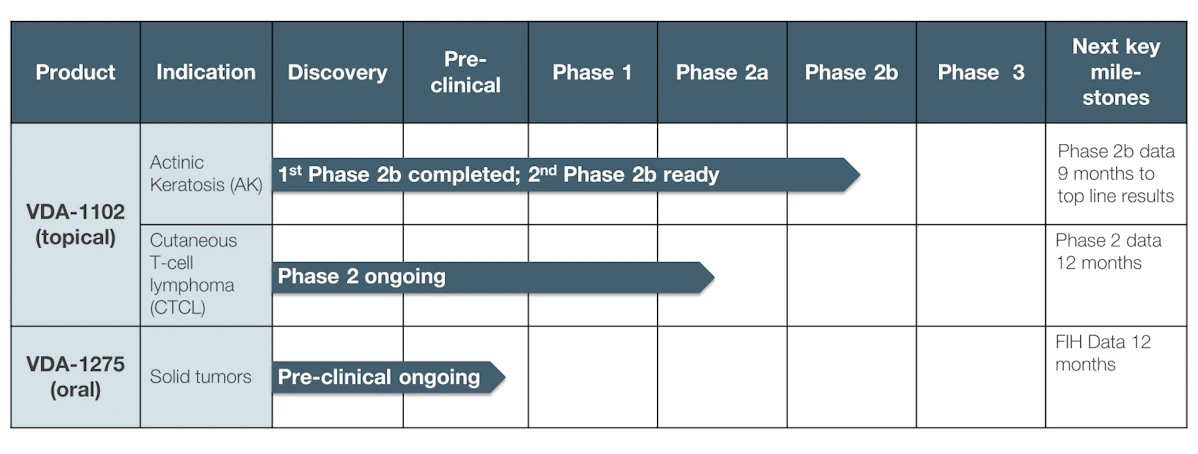
VDA-1102 - Actinic Keratosis (AK)
VDA-1102 ointment is the first in Vidac’s new class of cancer drugs, which selectively targets malignant cutaneous cells, with minimal effects on surrounding healthy skin.
The drug candidate utilizes Vidac’s novel mechanism of action, reversing the abnormal metabolism of cancer cells. The ointment has demonstrated significant efficacy, in both in vitro and in vivo models for actinic keratosis (AK), an early form of skin cancer that can develop into cutaneous squamous cell carcinoma ().
VDA-1102 is equally effective in reducing the lesions which develop in AK as drugs that are currently marketed, such as 5-FU and ingenol mebutate (Picato®). However, unlike these treatments, VDA-1102 causes a minimum of unwanted side-effects, as it targets tumor cells, not healthy skin cells. The drug causes neither necrosis nor a high inflammatory reaction - reasons why patients often avoid initial treatment and need repeat treatment - addressing a significant unmet medical need.
Clinical trial summary:
A Phase 2b clinical trial in AK (NCT 03538951), also known as non-melanoma skin cancer, demonstrated in a post hoc analysis a 40% complete clearance of lesions and 80% overall reduction of lesions. A second Phase 2b study is about to launch with only advanced AK patients, as the earlier study found higher sensitivity in these patients than in those with less severe, e.g. benign forms of the disease. The trial, against standard of care, will be done with up to 40 patients in each of its two arms, with endpoints the clearance of highly proliferative lesions and the progression of AK lesions progressing to cSCC.
VDA-1102 - Cutaneous T-Cell Lymphoma (CTCL)
The exceptional safety found in Phase 2b for VDA-1102 in AK, enabled Vidac to enter directly into a Phase 2 study for Cutaneous T-Cell Lymphoma (CTCL), a type of cancer of the immune system. Caused by a mutation in the T-cells, CTCL is a rare disease which causes lesions to appear in the skin, before the disease spreads through the body. An orphan disease, CTCL may be eligible for fast-track regulatory processing.
Clinical trial summary:
A Phase 2 trial is ongoing. Interim results from 50% of subjects in January of 2024 showed favorable results compared to the standard of care, which has a lower complete response rate and a much longer median response time. This is a Phase 2 open-label, within-subject vehicle-controlled study to evaluate the efficacy, safety, tolerability, and pharmacokinetics of VDA-1102 topical ointment application in adult subjects with relapsed stage-1 Mycosis Fungoides A.
The next member of Vidac’s family of potent small molecules, VDA-1275 has proven to be highly potent against a broad range of tumor types in in vitro studies. In February 2024, Vidac announced exceptionally strong results for VDA-1275 in multiple mouse cancer and human cellular organoid model of solid tumors. The drug candidate showed statistically significant efficacy as a monotherapy, as well as significant synergistic effects in combination with two standard-of-care cancer treatments. The study also showed that VDA-1275 induced an immunologic response by itself. VDA-1275, while chemically unrelated to VDA-1102, targets the same mode of action: uncoupling the binding of the hexokinase 2 (HK2) enzyme with the mitochondrial VDAC channels.
Clinical trial summary:
The company is developing VDA-1275 as a systemic drug to treat solid tumors, with First-in-Human trials expected to start in Q1 2025.

